Pediatric Hospital Crib | Safe, Adjustable, Easy-Clean
What hospitals really want in a Pediatric Hospital Crib in 2025
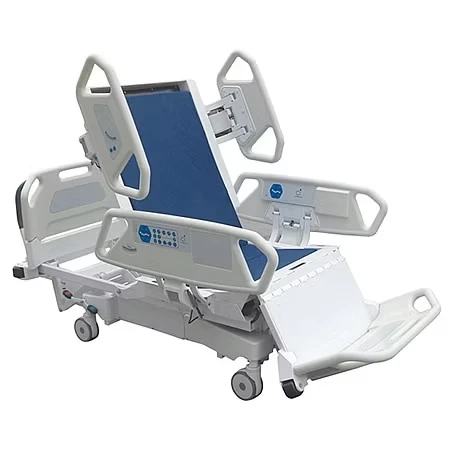
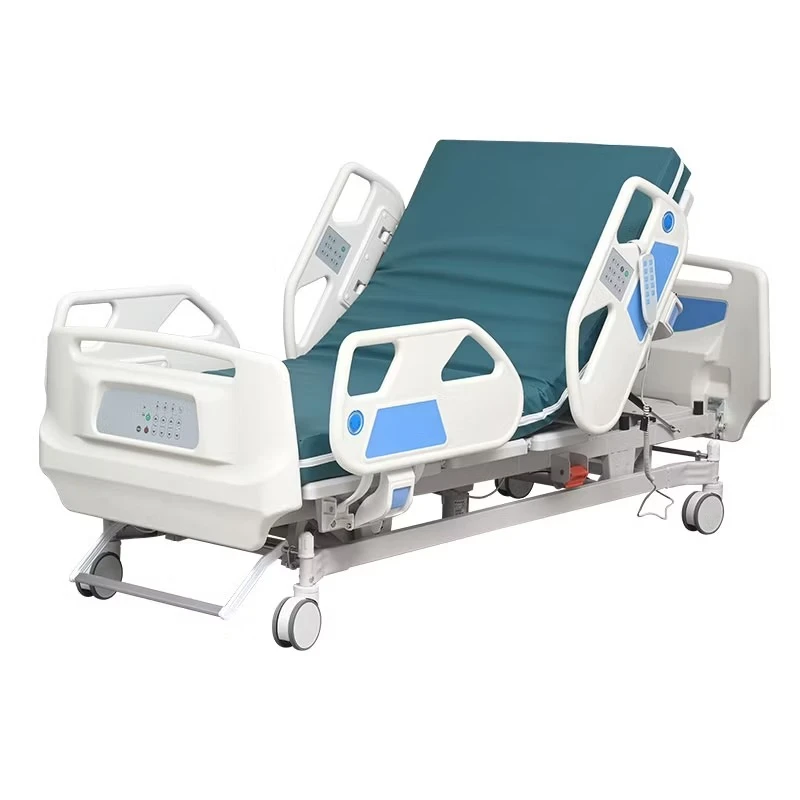
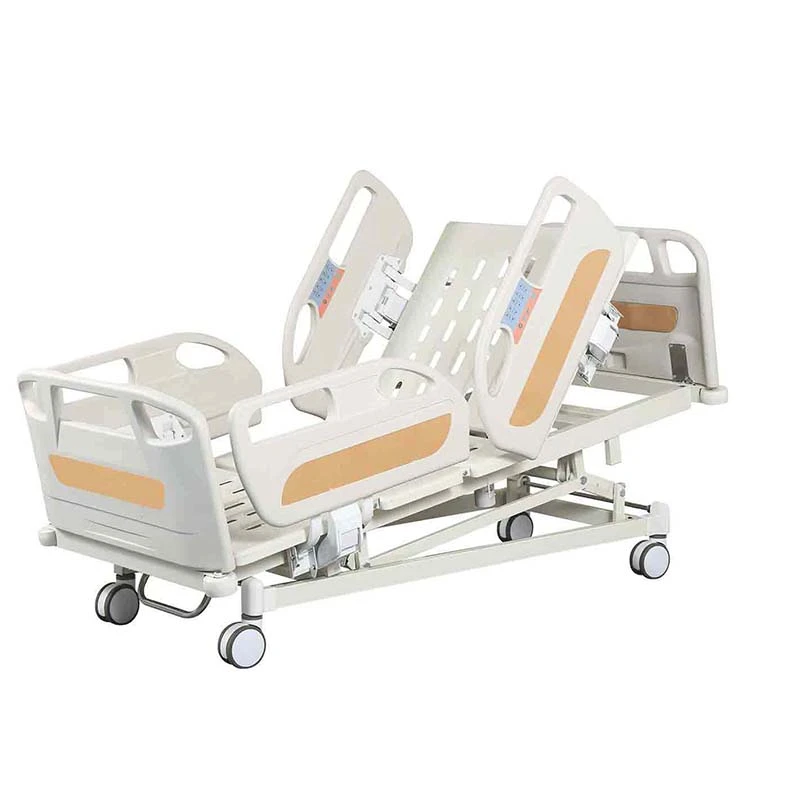
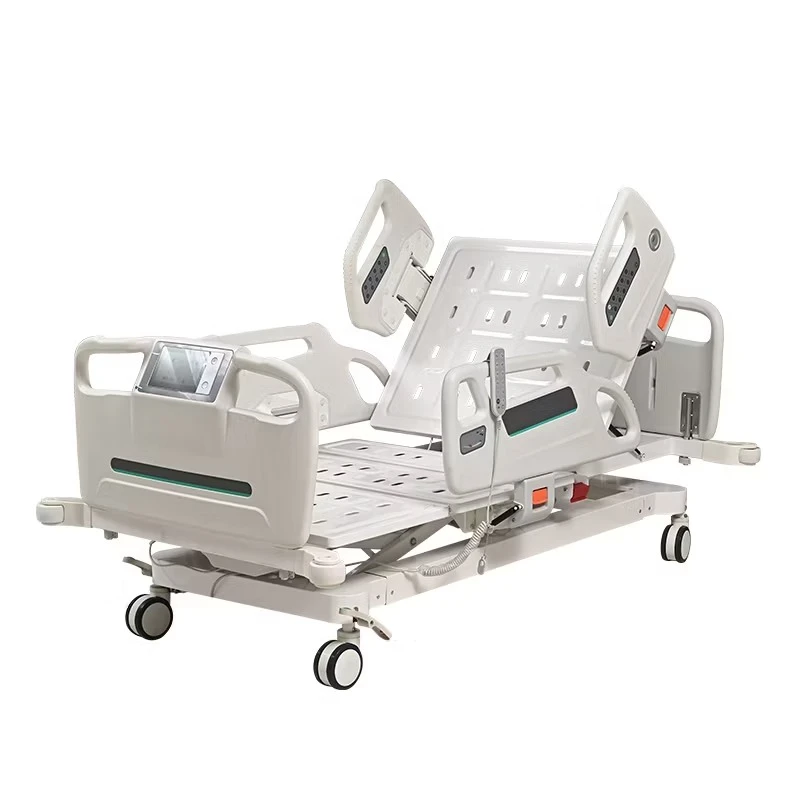
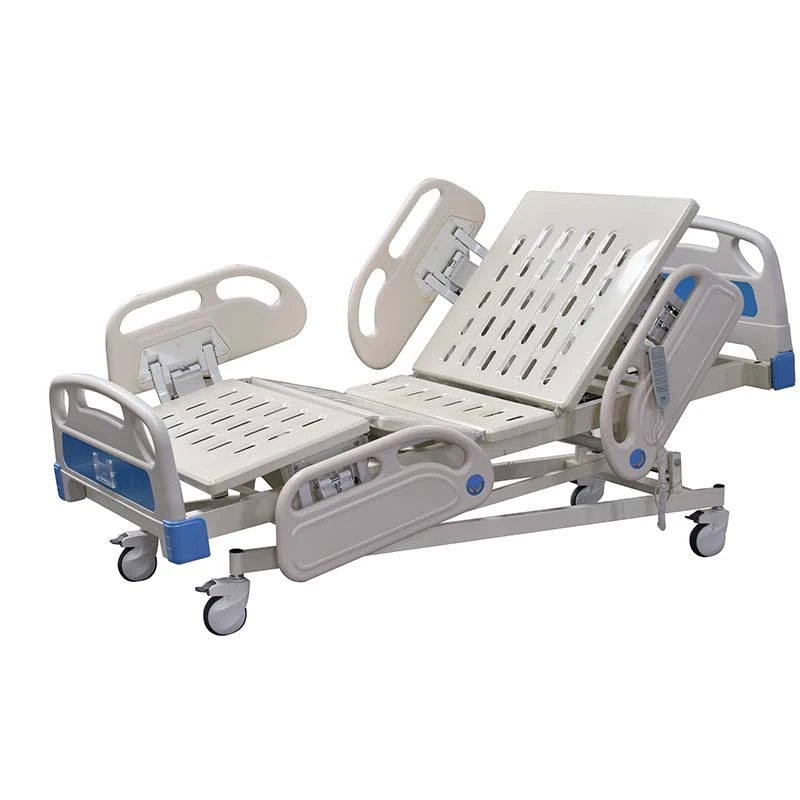
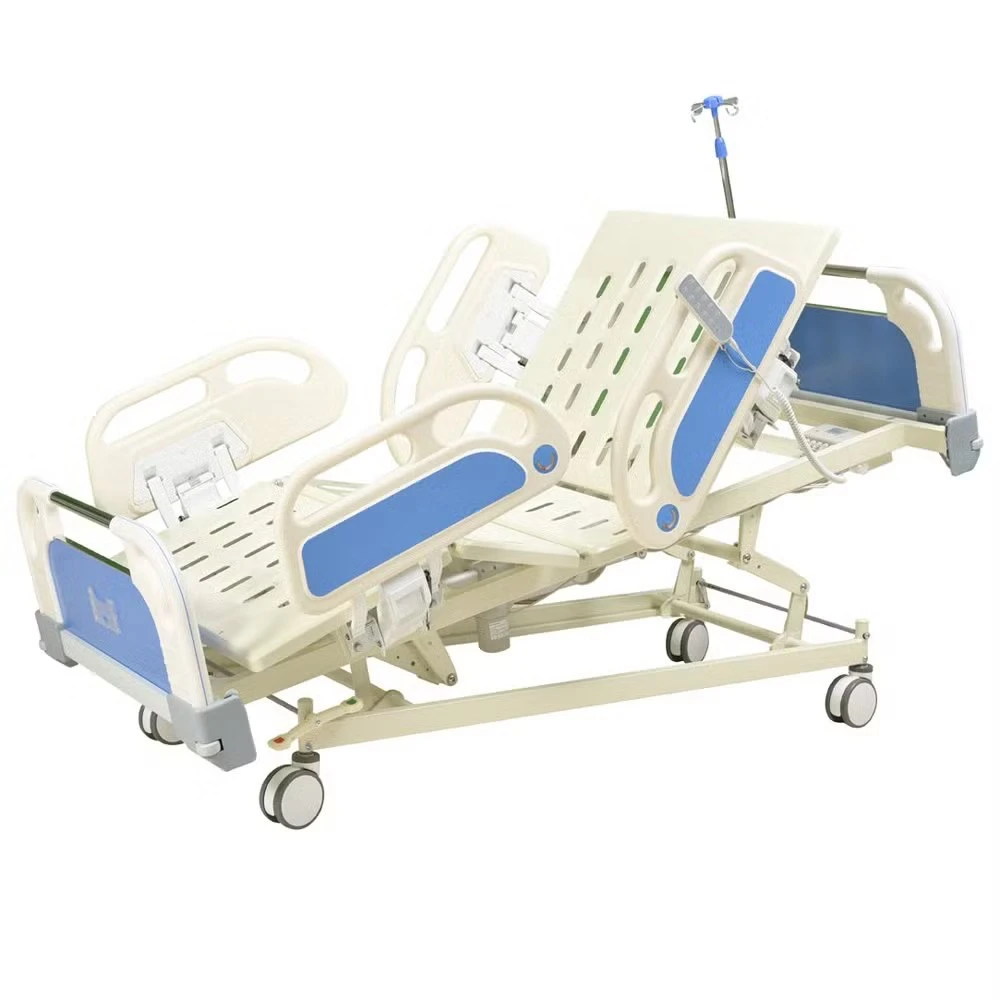
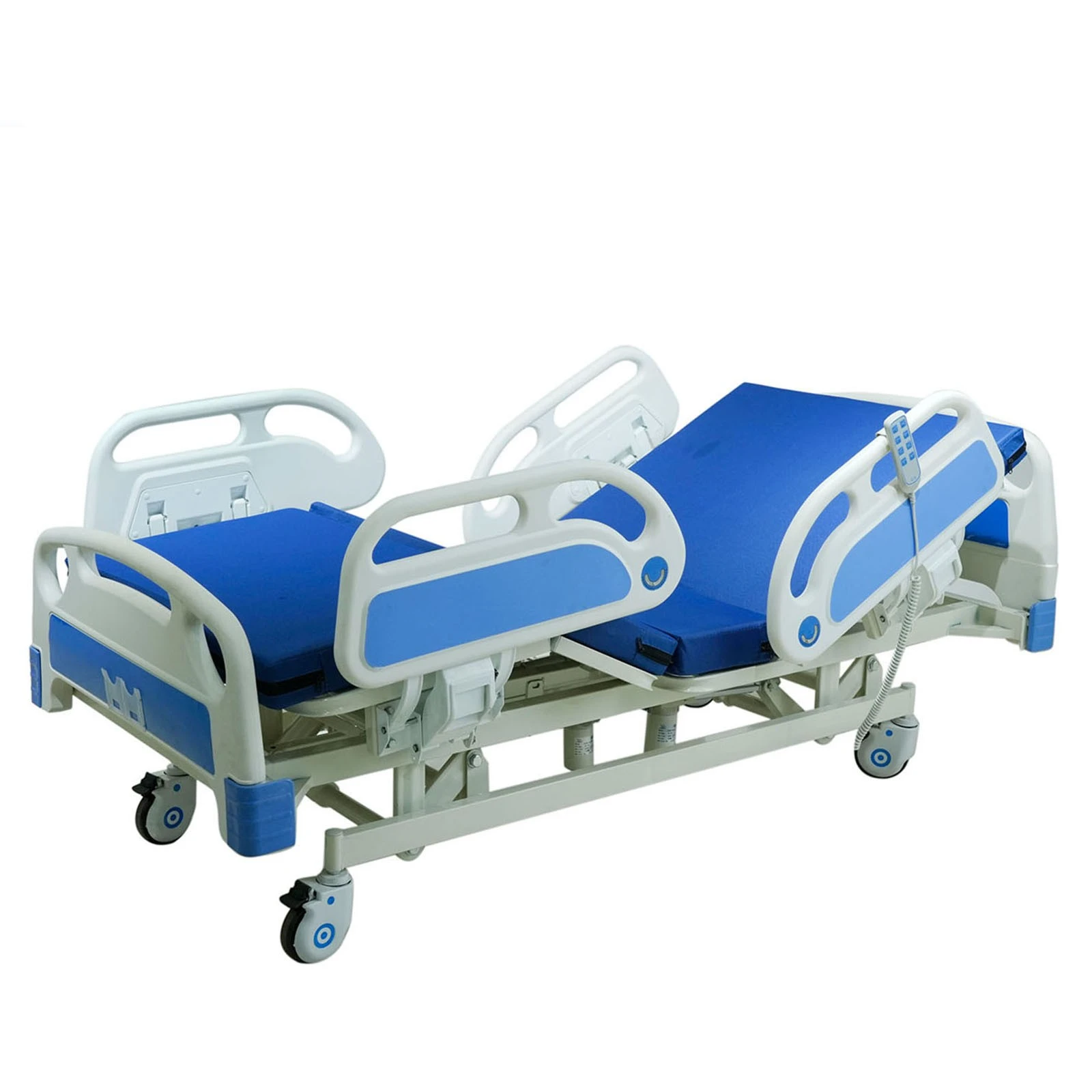
If you’ve spent a night on a postpartum ward (I have, too many times to count), you know: the humble bassinet does more than hold a newborn. It’s a mobility aid, a micro-care station, and honestly, a peace-of-mind device for nurses and parents. That’s why the Baby Hospital Bassinet 3 Functions caught my eye. Built in Zhouhu Village, Jizhou Zone, Hengshui City, Hebei Province, China, it leans into clinical practicality: Trendelenburg, reverse Trendelenburg, and whole-bassinet height adjustment. Simple, but meaningful.

Industry trend check
Hospitals are standardizing around tilt controls for reflux and phototherapy positioning, anti-microbial surfaces, and fewer maintenance points. To be honest, most teams want fewer buttons and more reliability. The Pediatric Hospital Crib category is also drifting toward ISO 13485–audited manufacturing and MDR-ready documentation, even when devices are largely mechanical.
Real-world use cases
-
- Postpartum rooming-in with quick height changes for C‑section recovery
- NICU step-down, gentle Trendelenburg for reflux management (per clinician protocol)
- L&D handoff and short in-ward transport with locking casters
- Night rounding: quiet casters and easy wipe-downs save time

Product snapshot: Baby Hospital Bassinet 3 Functions
| Spec | Details (≈ values; real-world use may vary) |
|---|---|
| Adjustment | Trendelenburg / Reverse Trendelenburg; gas-spring height adjustment |
| Tilt range | ≈ ±12° |
| Height range | ≈ 780–980 mm |
| Load rating | ≈ 20 kg |
| Materials | Powder-coated steel or aluminum column; transparent PC/ABS bassinet; TPU bumpers |
| Casters | Ø100 mm, two with brakes; low-noise, anti-static |
| Footprint | Compact base for bedside access; shelf for consumables |
| Service life | ≈ 8–10 years with routine maintenance |
Build process and testing
-
- Materials: sourced PC/ABS tub, welded steel frame, powder coat ≥70 μm
- Methods: TIG welding, CNC bending, gas-spring integration, torque-checked fasteners
- Testing: tilt cycle test ≈15,000 cycles; caster roll test ≈20 km; static load 1,000 N on frame; salt-spray 72 h on coated parts
- Standards referenced: IEC 60601-2-52 (medical beds—rails/entrapment principles), ISO 14971 (risk), ISO 10993-1 (biocompatibility for patient-contact plastics), ASTM F2194 (bassinets/cradles guidance)
- Cleaning: compatible with quats and 70% IPA; avoid chlorine >1,000 ppm
Vendor snapshot (what buyers compare)
| Vendor | Certs (claimed) | Lead time | Warranty | Notes |
|---|---|---|---|---|
| Zhaofa Medical | ISO 13485, CE (MDR) docs; FDA establishment (verify) | ≈ 20–30 days | 2 years | Strong value; customization options |
| Generic Importer A | Basic CoC; limited tech file | ≈ 45 days | 1 year | Lower price, minimal docs |
| EU Premium Brand | ISO 13485, CE MDR, full UDI | ≈ 6–8 weeks | 3–5 years | Top finish, highest price |
Customization and options
Hospitals often ask for color-matched powder coat (RAL), logo silkscreen, bassinet name-card holder, IV pole, and an under-shelf. The Pediatric Hospital Crib setup here supports those without overcomplicating procurement.
Case notes and feedback
A 200-bed county hospital in Hebei swapped 30 units; nurses reported quieter night rounds and fewer “where’s the brake?” moments—seems trivial until 3 a.m. A private birth center in the GCC told me the reverse Trendelenburg helped with mild reflux cases (as per their protocol), and parents liked the clear tub visibility. To be honest, the win is low drama: reliable tilt, clean lines, stable base.

Bottom line
If your checklist is sane ergonomics, documented testing, and straightforward maintenance, this Pediatric Hospital Crib design makes sense. As always, verify certificates, request test reports, and run a ward trial before bulk purchase.
References
- ASTM F2194 – Standard Consumer Safety Specification for Bassinets and Cradles.
- IEC 60601-2-52: Medical beds—Particular requirements for basic safety and essential performance.
- ISO 14971:2019—Application of risk management to medical devices.
- ISO 10993-1:2018—Biological evaluation of medical devices.
- WHO Baby-friendly Hospital Initiative, revised guidance 2018.